
Therefore, there are various non-equivalent definitions of atomic radius.
NAME OF NA ELEMENT FREE
However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. It must be noted, atoms lack a well-defined outer boundary. The atomic radius of Sodium atom is 166pm (covalent radius). Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. Mass numbers of typical isotopes of Sodium are 23. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. It occurs abundantly in nature in compounds, especially common salt sodium. Sodium is the most common alkali metal and the sixth most abundant element on Earth, comprising 2.8 percent of Earth’s crust. Sodium is a very soft silvery-white metal. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.įor stable elements, there is usually a variety of stable isotopes. sodium (Na), chemical element of the alkali metal group (Group 1 Ia) of the periodic table. Neutron number plus atomic number equals atomic mass number: N+Z=A. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Sodium is a chemical element with atomic number 11 which means there are 11 protons in its nucleus. Visit Bodycote’s new Interactive Periodic Table to view this information and more graphically.Atomic Number – Protons, Electrons and Neutrons in Sodium Chemical symbolįrom the Latin Argentum which originally meant shining metalįrom the Latin Aurum which originally meant yellowįrom Cuprum, the Latin name for Cyprus, the Roman source of copperįrom the Latin Hydragyrummeaning liquid silverįrom Natrium, the Latin namefor sodium carbonateįrom the Latin Plumbummeaning soft white metalįrom the Latin Stibiummeaning cosmetic powderįrom Wolfram, an old name for the element derived from its ore, Wolframite Visit Bodycote’s new Interactive Periodic Table to view this information and more graphically. In those cases, the origin of the symbol used is given. It is a depiction of the periodic law, which says that when the elements are arranged in order of. It is an organizing icon of chemistry and is widely used in physics and other sciences. A very few elements have symbols which appear to have no relationship with their names. The periodic table, also known as the periodic table of the elements, arranges the chemical elements into rows ('periods') and columns ('groups').
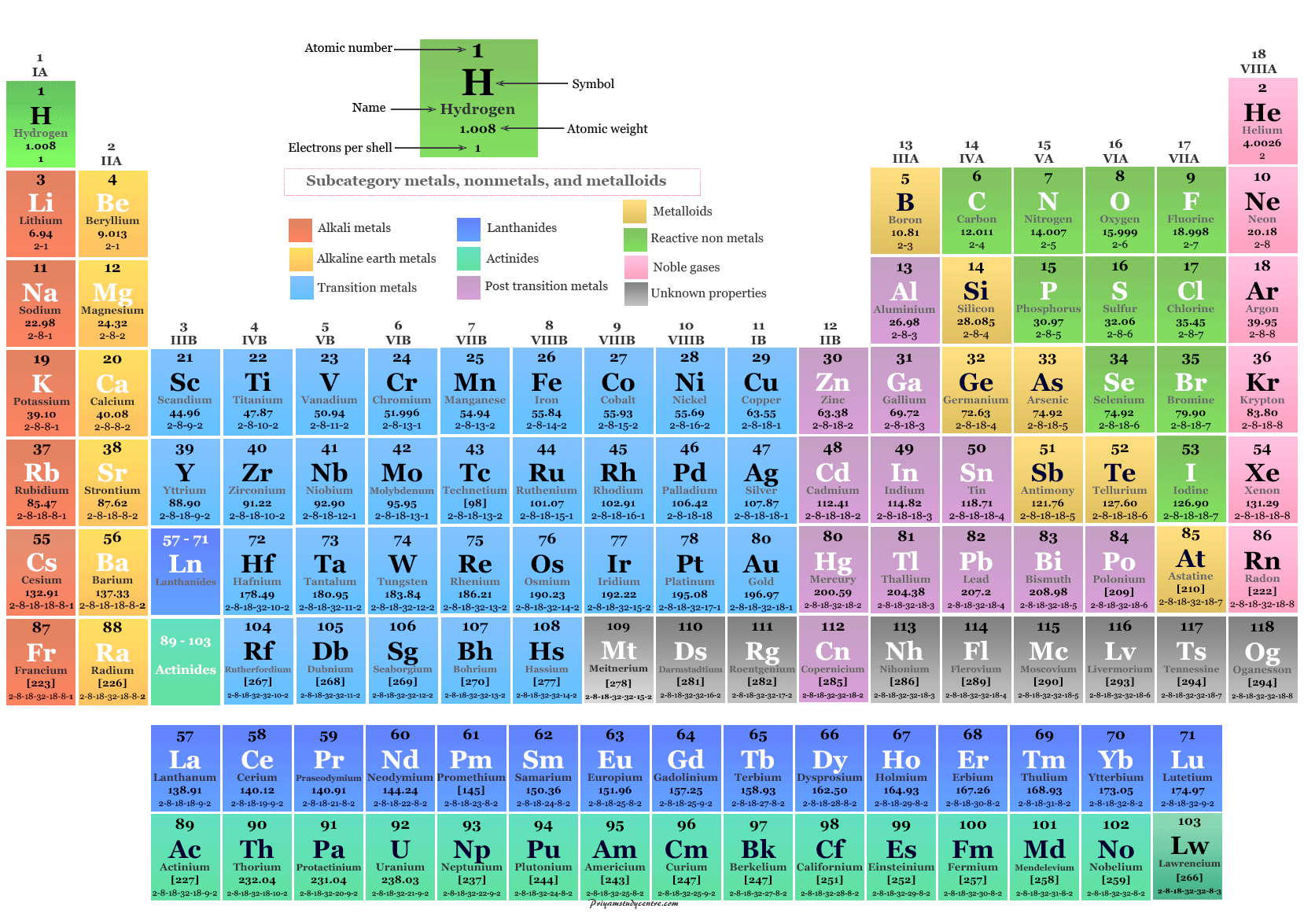
In some cases, the first letter together with some other letter from their name was used, particularly when their first two letters had already been allocated to another element. Visit BYJU’S to learn Symbols, Atomic Number, Atomic Mass, Groups, Videos with FAQs of Periodic Table Elements. In the periodic table, the vertical columns are called groups and the horizontal rows are called periods.

Most chemical elements are represented symbolically by two letters, generally the first two in their name. Periodic Table of Elements - The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. Transition Metals: Groups 3-12 - d and f block metals have 2 valence electrons. Alkaline Earth Metals: Group 2 (IIA) - 2 valence electrons. Powdermet® Selective surface net shape (SSNS)Īnti-Slavery and Human Trafficking Statement Another common method of categorization recognizes nine element families: Alkali Metals: Group 1 (IA) - 1 valence electron. Sub-critical annealing / intercritical annealing Specialty Stainless Steel Processes (S 3P) Precipitation hardening: Stainless steels Case hardening with subsequent hardening operationĬase hardening without subsequent hardening operationįluidised bed/salt bath nitriding/nitrocarburising Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds.


 0 kommentar(er)
0 kommentar(er)
